On May.31st.2021, The Ministry of Food and Drug Safety held an online civil complaint briefing session. The main purpose is to explain evaluation trends and also to provide supplementary cases for The registration of Individual functional ingredients.
1. Notified in the Codes of Health Functional Foods vs Individually recognized ingredients
1-1. Notified in the Codes of Health Functional foods (2021).
A manufacturer or distributor can use publicly notified functional ingredients for its health functional food product in accordance with the standards and specifications prescribed in the Health Functional foods. Currently, Nutrients (28) and functional ingredients (68) have been published in this code based on June 2021.
Mandatory Requirements; Health Functional Food Code:
Health Functional Food Code is to stipulate the standards and specifications of manufacture, processing, importation, distribution, and storage of health functional food products in South Korea. It includes:
● Chapter 1. General Provisions
● Chapter 2. General Standards and Specifications
● Chapter 3. Standards and Specifications for Each Food Category
● Chapter 4. Test method.
** Annexed the Table 5, Negative List for Health Functional Food Ingredients
Download: https://www.mfds.go.kr/eng/brd/m_15/view.do?seq=70011
1-2. Individually recognized ingredients by MFDA
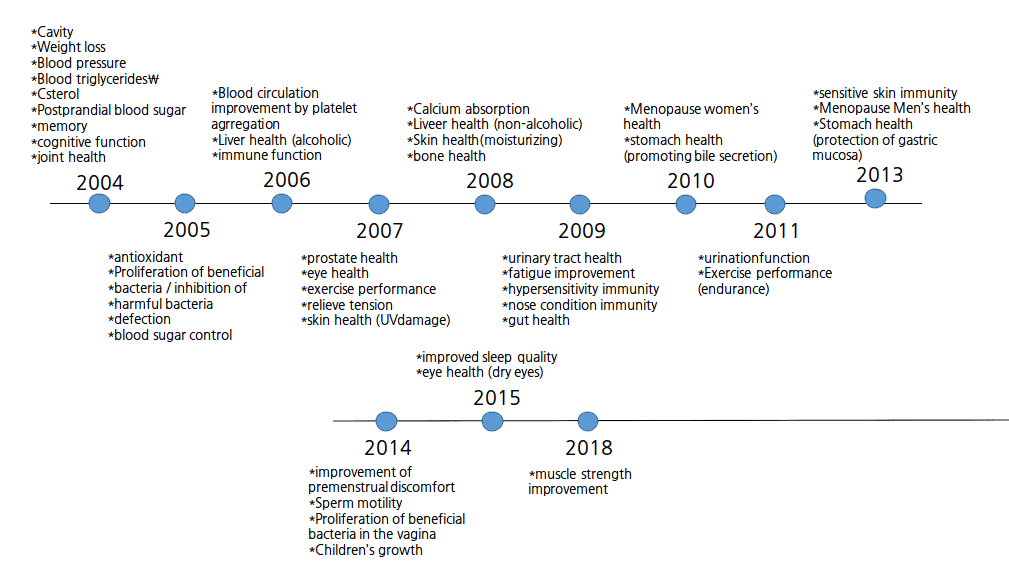
From 2004 to 2021 (May) total 245 ingredients are approved as the individual functional ingredients by MFDA. These ingredients can be converted to a notified ingredient when it is passed 6 years from the date of application and also It has more than 50 products manufacturing reports in South Korea.
The graphs of individual functional claims.
2. The improvement plan of health claims evaluation
The MFDA presented guidelines for registration of functional ingredients with strict standards, and also, along with the definition of regulatory science, suggested ways to overcome regulatory delays through communication with registrants.
2-1. Supplement for registration of functional ingredients
The most important thing to pay attention to is whether or not toxicity tests are added to prove safety.
1)Data on Raw materials
- Origin of raw materials and use part of raw materials
- Manufacturing record and manufacturing process chart (process charts include extract ratio, production yield, pH etc).
- Data that can confirm the prevention of mixing of similar raw materials and materials related to authenticity. (Genetic discrimination, morphological discrimination, functional compounds, etc.)
2)Data on Functional compounds
- Functional Compounds test and validation
- Pesticide residue
3)Supplement data on Safety
- Data that can evaluate intake amounts
- Toxicity studies; Single Dose Toxicity study, 90days Repeated dose toxicity study, genotoxicity-GLP toxicity study report.
- DB data information (toxicity, side effects); ECHA, Pubchem, NLM(Toxnet), Tox-info, etc.
Decision-making processes of Safety evaluation
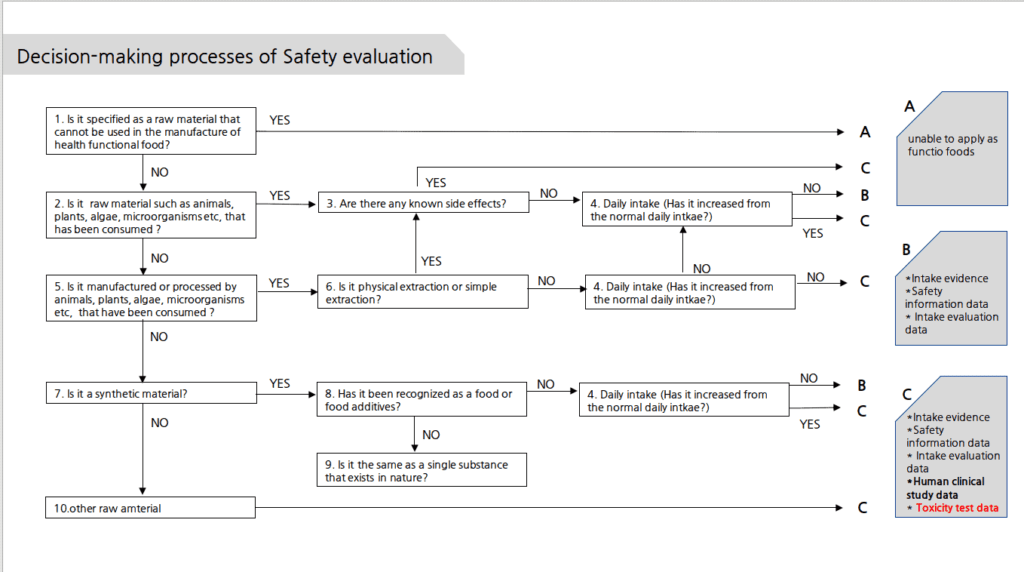
**4. Daily intake; The suggested intake of raw materials is 3times than the average intake of raw materials consumed on a daily basis (over 25years) or make sure it’s more than an extreme amount (95%)
4) Clinical study
- Description of adverse reactions identified in clinical studies.
- Data on the validity of study design, implementation, statistical processing results report, etc.
2-2. Regulatory science at functional food registration.
1) Definition of Regulatory Science
Regulatory science is the application of the scientific method to improve the development, review, and oversight of new drugs, biologics, and devices that require regulatory approval prior to dissemination. “
“the intellectual and practical activity encompassing the systematic study of the structure and behavior of the regulatory world through observation and experiment to determine the impact of the rules, principles, and laws governing FDA-regulated research.” -USA FDA
Accordingly, the MFDA job is to conduct regulatory management therefore, the relevance to regulatory science is increasing. And also, to promote the basic plan for promotion of Food and Drug safety Technology (2021-2025), MFDA seems likely to accept the concept of regulatory science.
MFDA is expected to actively accept regulatory science and propose strict standards for functional ingredient registration. Therefore, The MFDA service of “New Functional Prior Consultation Procedure ” will be more activated and important before the registration starts.
Those wishing to register should be able to conduct studies in accordance with strict standards management. Through this service, we are able to check how successful the studies which one will apply to functional ingredients have been at achieving its original purpose.


