MFDS(Ministry of Food and Drug Safety) announced new guideline of functional claims; Respiratory health and gums health
MFDA has prepared a functional evaluation guideline for health functional foods that can help with respiratory ( trachea*bronchiole) health and gums health.
The new functional claims are considered environmental changes such as fine dust and consumer interest and demand based on arising the aging of the population. The new guideline includes Mechanism of action, Major evaluation index (Biomarker), description and measurement methods for each biomarker and subjects of human application test for considerations test design.
According to the announcement from the MFDS, the joint analysis of 2019 clinical trial authorization landscape showed that the number of authorizations totalled 714 cases, a 5.2% increase compared to that of 2018 (697 cases). Among these studies, The main trends of Clinical trial was
▲steady rise in the number clinical trial authorizations
▲burgeoning of phase III clinical trials in Korea
▲growth in the number of clinical trials on diseases affecting the central nervous system, the respiratory system, and the cardiovascular system.
Especially, 23 clinical trials were conducted last year on the respiratory system. Many people are waiting for the respiratory functional food ingredients that proved functionality and safety based on studies.
MFDS expect new functional claims that improve the condition of slight chronic cough caused by environmental factors or improve the inflammation of airways and bronchi in asthmatics ordinary people.
Not only in Korea, Worldwide, Respiratory diseases appear in the top ten leading causes of death. Specifically, COPD (Chronic obstructive pulmonary disease).
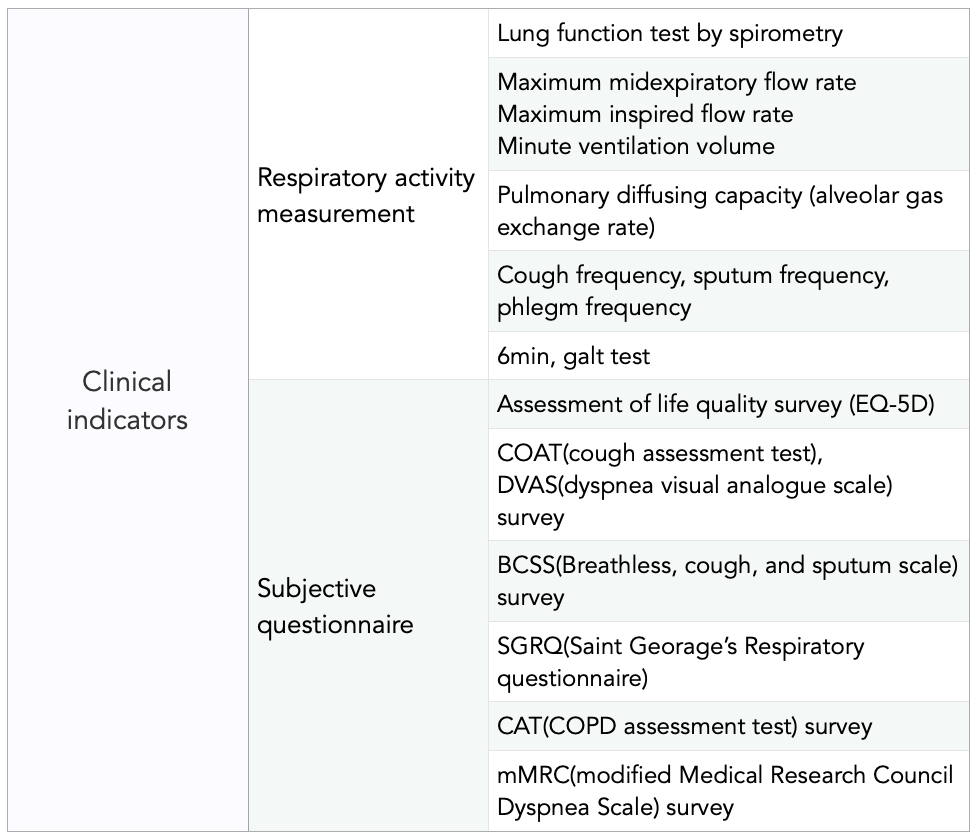
Throughout the life cycle, diet and lifestyle are important modifiable risk factors in the development, progression and management of obstructive lung disease, such as asthma and COPD. And Inflammation seems to be the leading contributor toward the progression of lung diseases. And MFDA suggests the Biomarker based on these factors.
[Biomarker for Clinical trial of respiratory health]
Monteloeder may help to improve your lifestyle and health by Zeropollution.
Zeropollution® began as a study to protect the skin from fine dust(environment factors), but continuously we are studying about respiratory health through more intensive ways based on correct inspection criteria as a special formulated ingredient.
Monteloeder may consider in the future to apply for health claims related to respiratory health by Zeropollution based on good experience about the registration of individual functional claims. We hope you pay close attention to Zeropollution from now on. And We are welcome to join the investment of Zeropollution® for Respiratory health studies in South Korea. .
Reference
Jane Lee – Korea General Manager


